ADDITIONAL DATA
- RaLu is a retrospective medical chart review of 133 patients from German Nuclear Medical centers who received Xofigo →
Taxane → 177Lu-PSMA vs Taxane → Xofigo → 177Lu-PSMA therapies1 - RaLu is a retrospective medical chart review of 133 patients from German Nuclear Medical centers who received Xofigo → Taxane → 177Lu-PSMA vs Taxane → Xofigo → 177Lu-PSMA therapies1
- 13 patients who received taxane both before and after Xofigo were included in both groups
- 71% received 6 Xofigo injections
- 56% of patients received ≥4 life-prolonging therapies, including abiraterone (71%), enzalutamide (70%), docetaxel (74%) before starting 177Lu-PSMA
- 73% of patients received 1–4 177Lu-PSMA cycles and 27% received ≥5 cycles
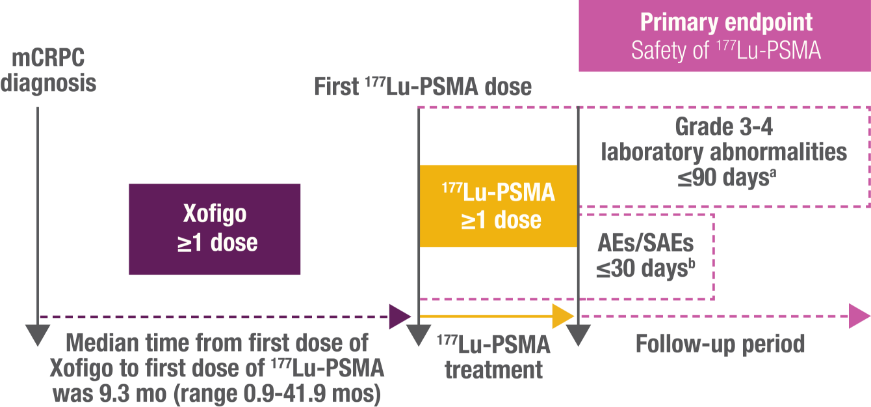
Study Limitations: Chart review studies have bias related to treatment selection and unreported variables cannot be fully addressed. Outcomes are based on clinical judgment, with variability in patient and adherence that can result in different outcomes, therefore no conclusions can be drawn.
KEY INCLUSION CRITERIA
Eligible patients were males aged ≥18 years, with bone metastases and a confirmed diagnosis of mCRPC, who had received ≥1 dose of Xofigo and, in any subsequent therapy line, ≥1 dose of 177Lu-PSMA
Data were retrospectively collected from Sep 2021 – Mar 2022.
aMeasured from start of 177Lu-PSMA therapy up to 90 days after last administration.
bMeasured from start of 177Lu-PSMA therapy up to 30 days after last administration.
AEs, adverse events; mCRPC, metastatic castration-resistant prostate cancer; SAEs, serious adverse events; TEAEs, treatment-emergent adverse events; 177Lu-PSMA.
Reported TEAEs and Lab Abnormalities During and Post 177Lu-PSMA Treatment
REPORTED TREATMENT-EMERGENT ADVERSE EVENTS
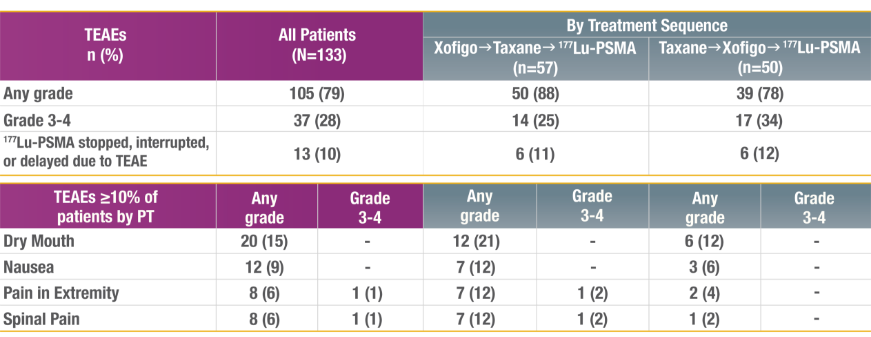
Measured from the start of 177Lu-PSMA up to 30 days after last dose
REPORTED GRADE 3-4 LABORATORY ABNORMALITIES
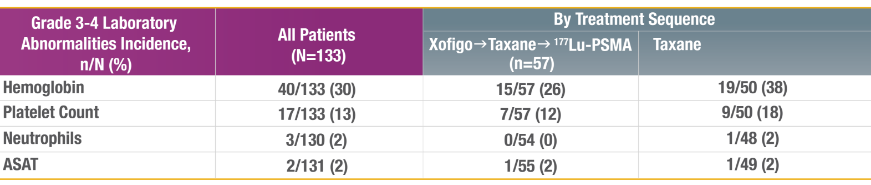
ASAT, aspartate aminotransferase; N, number of patients evaluated; n, number of patients with the specified event; PSMA,
prostate-specific membrane antigen; Tax, taxane-based chemotherapy; TEAE, treatment-emergent adverse event.
Measured from the start of 177Lu-PSMA up to 30 days after last dose
Reference
- Rahbar K, et al. Presented at European Society for Medical Oncology Annual Meeting; September 9-13, 2022; Paris, France. Return to content

