Other Endpoints: Quality of Life Measures from ALSYMPCA
FACT-P TOTAL SCORE IN COMPARISON TO EQ-5D UTILITY SCORE
CHANGES IN FUNCTIONAL ASSESSMENT OF CANCER THERAPY-PROSTATE (FACT-P) TOTAL SCORE AND EUROQOL UTILITY SCORE POST-HOC ANALYSIS1,a
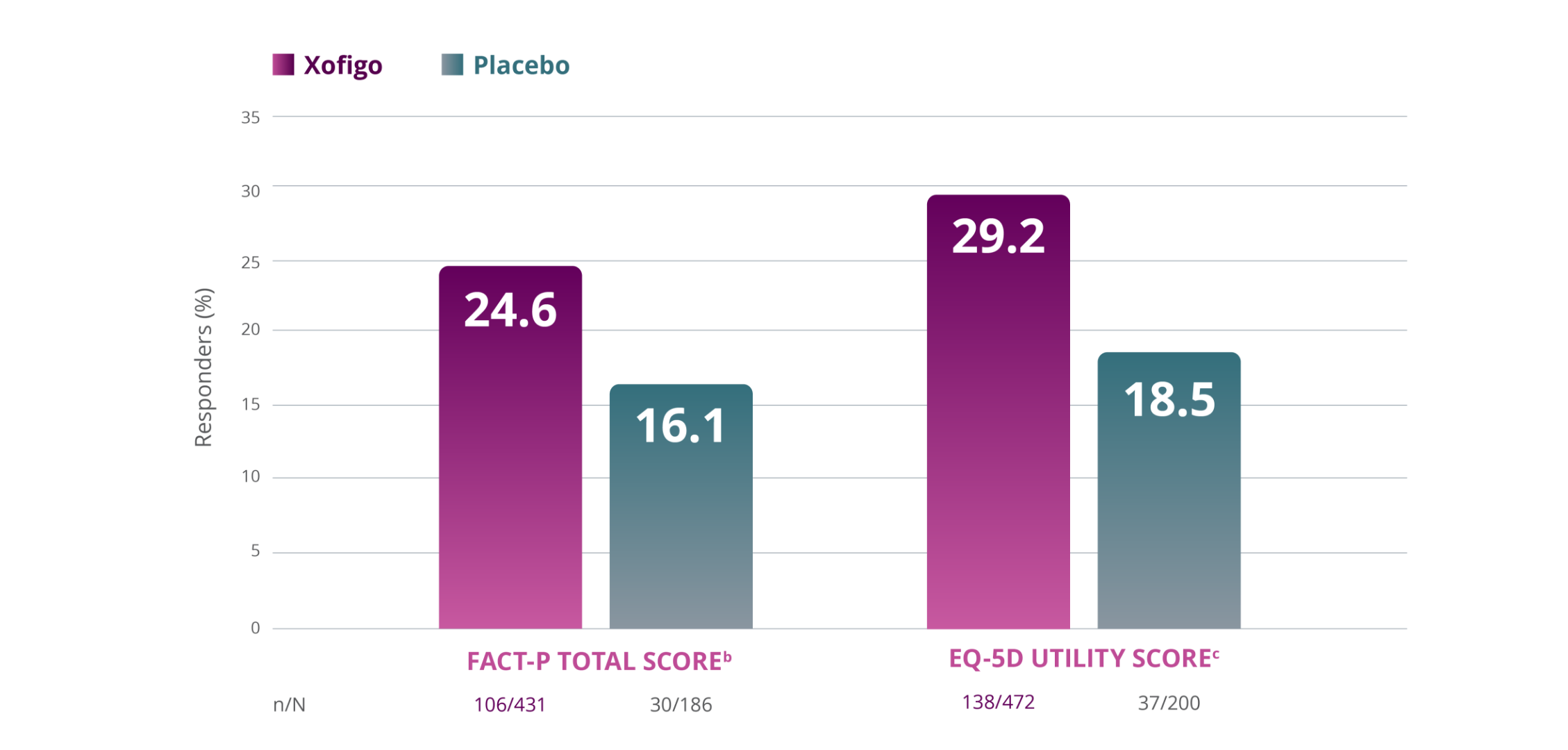
- Changes in FACT-P and EQ-5D were exploratory endpoints in the ALSYMPCA trial. These endpoints were not powered to detect treatment differences in patients’ QoL
Adapted from Nilsson, et al.
aPost-hoc analysis from ALSYMPCA.
bA responder was defined as a patient having an increase in FACT-P ≥10 from baseline at Week 16 and/or Week 24.2
cA responder was defined as a patient having an increase in EQ-5D utility score of ≥0.1 from baseline at Week 16 and/or Week 24.2
Reference
- Nilsson S, Cislo P, Sartor O, et al. Patient-reported quality-of-life analysis of radium-223 dichloride from the phase III ALSYMPCA study. Ann Oncol. 2016;27(5):868-874. Return to content

